Abstract
Background: Allogeneic stem cell transplantation (alloSCT) can cure high-risk myeloid malignancies. While non-relapse mortality has declined over the last several decades, more than half of alloSCT recipients die from recurrent leukemia, and little progress has been made in preventing or treating it. Only 10-20% of subjects with relapsed leukemia post-alloSCT become long-term survivors despite receiving donor lymphocyte infusions (DLI) or a second alloSCT. In mouse models, we identified that optimal CD4- and CD8- mediated graft-versus-leukemia (GVL) responses require interferon-gamma receptor (IFN-γR) expression on myeloblastic leukemias (Matte-Martone and Shlomchik, J Clin Invest. 2017). Other groups reported that IFN-γ restores HLA expression on myeloid blasts from patients who relapsed post-alloSCT. These data support the hypothesis that IFN-γ sensitizes myeloid leukemia cells to alloreactive T cell-mediated killing. We conducted a phase I trial to evaluate whether a standard dose of IFN-γ can safely be administered to patients with relapsed acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) post-alloSCT (NCT04628338).
Methods: This investigator-initiated trial aimed to evaluate 1) the feasibility and safety of IFN-γ post alloSCT as monotherapy and combined with DLI; and 2) whether the standard dose IFN-γ is sufficient to upregulate IFN-γ-inducible molecules in myeloblasts in vivo. Major eligibility criteria included: 1) HLA-matched alloSCT recipient with relapsed AML/MDS; 2) measurable bone marrow (BM) myeloblasts (0.5-30%) by flow cytometry, 3) no increases in systemic immunosuppression for 4 weeks; and 4) no history of grade IV graft-versus-host disease (GVHD). The study had two stages: IFN-γ monotherapy (stage 1) with a fixed dose of IFN-γ (ACTIMMUNE®, Horizon Therapeutics) 100 mcg subcutaneously 3 times per week (outpatient self-injection) for 4 weeks and subsequent DLI combined with IFN-γ (stage 2) for 8 more weeks. Clinical responses were evaluated by BM pathology and donor chimerism at 4 weeks (pre-DLI), 12 weeks, and 6 months after the first dose of IFN-γ. BM, peripheral blood mononuclear cells, and plasma were collected before, 48-60 hours after IFN-γ, pre-DLI, and post-DLI. Phosphorylated STAT1 and HLA-ABC/DR, ICAM-1, and PDL-1 expression on BM leukemia blasts were measured by flow cytometry.
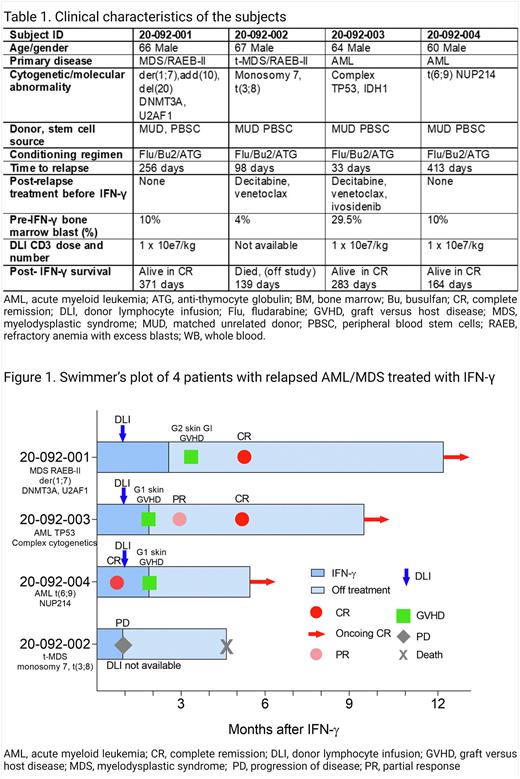
Results: Four patients with post-transplant relapsed AML/MDS were enrolled (Table 1). All patients tolerated IFN-γ monotherapy well with anticipated injection site reactions, flu-like symptoms, and stable cytopenia. Three patients who received DLI achieved complete molecular and cytogenetics remissions and conversion to full donor chimerism coincident with steroid-sensitive GVHD. One of the three responders with donor T cell chimerism of >90% pre-treatment achieved a CR with IFN-γ monotherapy before DLI (Figure 1). All responders are currently alive and in complete remission without requiring additional anti-leukemia maintenance therapy (median follow-up 283 days; range 164-371). One patient who did not have available DLI and had poor donor T cell chimerism (8%) died from disease progression on day 139. BM blasts from pre-treatment phosphorylated pSTAT1 in response to in-vitro IFN-γ, demonstrating intact upstream IFN-γ R signaling. Blasts from all four patients increased HLA-DR expression 48-60 hours after IFN-γ therapy in vivo. We are performing single-cell RNAseq of BM cells to better understand IFN-γ -induced transcriptome changes across different maturation stages of leukemia blasts and donor-derived normal hematopoietic cells.
Conclusion: IFN-g was safe in this pilot cohort of subjects with relapsed AML/MDS post-alloSCT. To our knowledge, this is the first time that IFN-γ has been tested on such subjects. In vivo, IFN-γ induced the predicted changes in myeloblasts. Most importantly, we observed durable CRs and conversion to 100% donor hematopoiesis with the IFN-γ/DLI combination, which is very promising for such poor-risk patients. These data provide a strong rationale for developing a phase II trial of IFN-γ/DLI for post-alloSCT relapse, and we are currently planning such a study.
Disclosures
Ito:Horizon Therapeutics: Other: Donation of study drug to the clinical trial ; Blue Sphere Bio: Patents & Royalties, Research Funding. Krakow:HighPass Bio: Research Funding. Hill:Applied Molecular Transport: Research Funding; Commonwealth Serum Laboratories: Consultancy; Compass Therapeutics: Research Funding; Cynata Therapeutics: Consultancy; iTeos Therapeutics: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Generon Corporation: Consultancy; NapaJen Pharma: Consultancy; Heat Biologics: Research Funding; Neoleukin Therapeutics: Consultancy; Serplus Technology: Research Funding; Genentech: Research Funding; Laevoroc Oncology: Research Funding. Shlomchik:BlueSphere Bio: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Currant insights: Consultancy; Rheos Medicines: Consultancy.
OffLabel Disclosure:
Interferon-gamma (Actimmune) for relapsed AML or MDS after allogeneic stem cell transplantation
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal